Spinal Fusion Bone Graft Substitutes Market Dynamics, Competitive Landscape, Outlook 2025-2032
The report thoroughly covers the profit margins of key players across different regions and segments, providing insights into the financial performance of industry stakeholders.
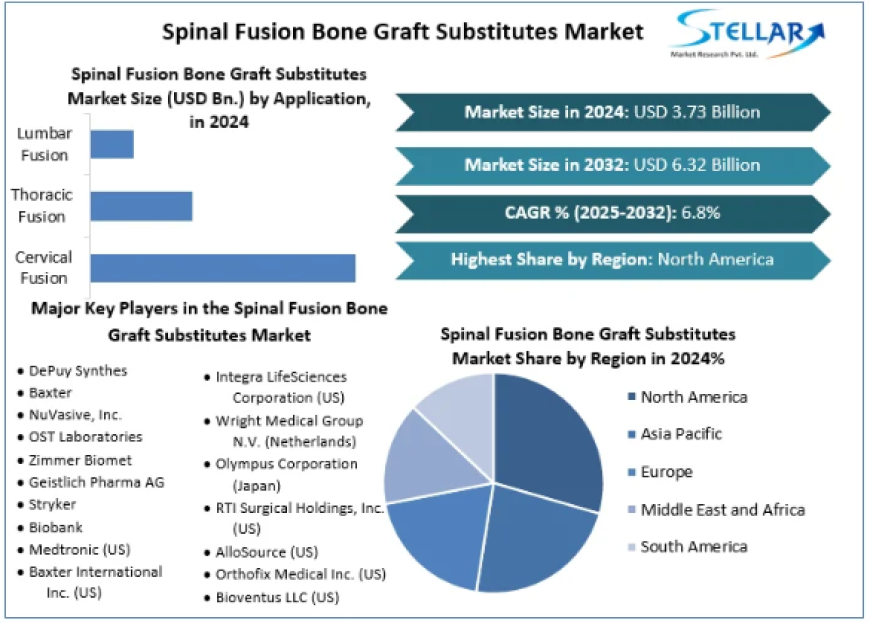
Spinal Fusion Bone Graft Substitutes Market size was valued at USD 3.73 Bn. in 2024 and the total Global Spinal Fusion Bone Graft Substitutes revenue is expected to grow at a CAGR of 6.8% from 2025 to 2032, reaching nearly USD 6.32 Bn. by 2032.
Market Definition and Estimation
Spinal fusion bone graft substitutes are biological or synthetic materials used to facilitate bone growth and fusion in spinal surgeries, serving as alternatives to traditional autografts. These substitutes play a crucial role in treating various spinal conditions, including degenerative disc disease and spinal deformities. The market's valuation at USD 3.73 billion in 2024 underscores the increasing demand for advanced spinal fusion solutions.
Get your sample copy of this report now! https://www.stellarmr.com/report/req_sample/Spinal-Fusion-Bone-Graft-Substitutes-Market/1783
Growth Drivers and Opportunities
Several factors are propelling the growth of the spinal fusion bone graft substitutes market:
-
Technological Advancements: Innovations such as bioresorbable materials, engineered bone grafts, and bone morphogenetic proteins (BMPs) have enhanced the efficacy of spinal fusion procedures, leading to improved patient outcomes and reduced recovery times.
-
Rising Preference for Minimally Invasive Surgeries (MIS): MIS techniques offer benefits like reduced postoperative pain and shorter hospital stays, increasing the demand for compatible bone graft substitutes.
-
Aging Population: The growing geriatric population is more susceptible to spinal disorders, thereby increasing the need for spinal fusion surgeries and associated graft substitutes.
-
Favorable Reimbursement Policies: In regions like North America, supportive reimbursement frameworks facilitate patient access to advanced spinal treatments.
Segmentation Analysis
The spinal fusion bone graft substitutes market is segmented based on type and application:
-
By Type:
-
Autografts: Bone grafts harvested from the patient's own body.
-
Allografts: Donor bone grafts obtained from a different individual.
-
Xenografts: Bone grafts derived from a different species.
-
-
By Application:
-
Cervical Fusion: Fusion procedures in the neck region.
-
Thoracic Fusion: Fusion procedures in the upper and mid-back.
-
Lumbar Fusion: Fusion procedures in the lower back.
-
To delve deeper into this research, kindly explore the following link: https://www.stellarmr.com/report/Spinal-Fusion-Bone-Graft-Substitutes-Market/1783
Country-Level Analysis: USA and Germany
United States:
The U.S. remains a dominant player in the spinal fusion bone graft substitutes market, attributed to its advanced healthcare infrastructure and high prevalence of spinal disorders. Annually, over 200,000 spinal fusion and 500,000 bone graft procedures are performed in the country. The adoption of innovative surgical techniques and favorable reimbursement policies further bolster market growth.
Germany:
Germany stands as a significant market within Europe, driven by its robust medical research ecosystem and emphasis on orthopedic innovations. The country's aging population and increasing incidence of degenerative spinal conditions necessitate advanced spinal fusion solutions. Germany's commitment to precision medicine and patient-specific treatments enhances the adoption of cutting-edge bone graft substitutes.
Competitive Landscape
The spinal fusion bone graft substitutes market is characterized by intense competition, with key players focusing on product innovation and strategic collaborations:
-
Orthofix Medical Inc.: In October 2023, the company launched OsteoCove™, a bioactive synthetic graft approved by the FDA, available in putty and strip formats, enhancing bone-forming properties for various orthopedic and spine surgeries.
-
NuVasive, Inc.: In February 2023, NuVasive received FDA clearance for its Modulus Cervical interbody implant, expanding its C360 product range.
-
Medtronic: In June 2022, Medtronic obtained FDA clearance for a ligament-augmenting implant, broadening its spine surgery portfolio.
-
Zimmer Biomet: In March 2022, Zimmer Biomet finalized a distribution agreement with Biocomposites for the supply of Genex bone graft substitutes.
Other prominent players include DePuy Synthes (Johnson & Johnson Inc.), Stryker, Baxter International Inc., and Integra LifeSciences Corporation.
Discover What's Trending :
North America Outpatient Surgical Procedures Market https://www.stellarmr.com/report/North-America-Outpatient-Surgical-Procedures-Market/698
North America Microtome Market https://www.stellarmr.com/report/North-America-Microtome-Market/700
Conclusion
The spinal fusion bone graft substitutes market is on an upward trajectory, fueled by technological advancements, a growing aging population, and increasing adoption of minimally invasive surgical techniques. With significant contributions from countries like the USA and Germany, and a competitive landscape marked by continuous innovation, the market is set to achieve substantial growth through 2032.
About Stellar Market Research:
Stellar Market Research is a global leader in market research and consulting services, specializing in a wide range of industries, including healthcare, technology, automobiles, electronics, and more. With a team of experts, Stellar Market Research provides data-driven market insights, strategic analysis, and competition evaluation to help businesses make informed decisions and achieve success in their respective industries.
For more information, please contact:
Stellar Market Research:
S.no.8, h.no. 4-8 Pl.7/4, Kothrud,
Pinnac Memories Fl. No. 3, Kothrud, Pune,
Pune, Maharashtra, 411029
+91 20 6630 3320, +91 9607365656

 Surekhammr
Surekhammr